There's a saying that "the shape of the still determines a distillery’s style," but while equipment designs can be replicated, the essence lies entirely in expertise. Simply guessing flavor profiles based on appearance may lead to conclusions as unreliable as the parable of the blind men and the elephant. Let’s explore how distillation techniques interconnect to influence the quality of spirits in century-old distilleries!
In any distillery, the golden gleam of the stills never fails to impress. Their diverse forms—tall and slender, short and stout, gracefully curved, bulbous yet flat-topped, or distinguished by towering necks—serve as iconic emblems of each distillery’s unique flavor profile. But before delving into the finer details of still design, one critical question must be addressed: Why copper?
Beyond its excellent malleability, thermal conductivity, and corrosion resistance, copper’s most vital role is its ability to remove impurities and refine the spirit's character. During fermentation, yeast converts compounds from grain into various congeners, including sulfides, imparting notes of boiled cabbage, sulfur, or rotten eggs—unpleasant flavors for most palates. However, these off-notes are neutralized through interaction with copper during distillation. Thus, still shapes are meticulously designed to control how long vapor or liquid alcohol remains in contact with copper walls.
For instance, shorter stills allow alcohol vapors to be collected quickly, minimizing copper contact and resulting in a bolder, more complex spirit with lingering impurities. Conversely, a swan neck with a bulbous design—like those at Ballindalloch—encourages vapor expansion, condensation, and reflux (where liquid flows back for re-evaporation), yielding a cleaner, smoother spirit. Additionally, copper acts as a catalyst, transforming pungent thiols into milder carbonyl compounds, though it has minimal effect on ester formation and slightly reduces phenolic content during distillation.

From a macro-physical perspective, all substances transition between solid, liquid, and gaseous states due to changes in temperature and pressure. Distillation harnesses this principle: applying heat converts the liquid wash into vapor, which is then condensed back into a liquid—now with a different composition, as separation depends on varying boiling points.
Fermented wash typically contains:
Water: ~86–94%
Alcohol: ~6–14%
Volatile congeners (flavor compounds carried by vapor): ~0.1%
Non-volatiles (undistillable solids): Trace amounts
After distillation, the new-make spirit comprises:
Water: ~35–5%
Alcohol: ~65–95%
Congeners: ~0–0.5%
The graph below illustrates alcohol concentration (% by volume) relative to temperature, demarcated into liquid, vapor-liquid, and vapor phases by two curves.
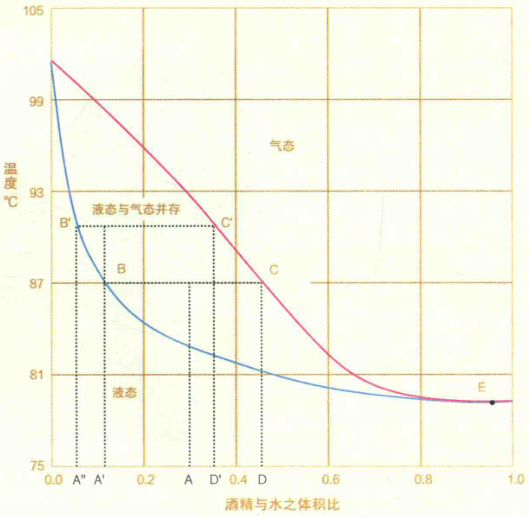
Since alcohol boils at a lower temperature than water, the vapor phase is always richer in alcohol than the original liquid. As distillation progresses, the wash’s alcohol content declines, raising the boiling point while both vapor and condensed alcohol concentrations diminish. When vapor alcohol dips to 1%, the wash retains merely 0.1% alcohol. Extracting further traces demands excessive energy, making continued distillation economically unviable—hence most distilleries halt the process here.
However, the wash isn’t just water and alcohol; trace compounds introduce added complexity to temperature-concentration curves. Yet the foundational theory remains the same, empowering distillers to fine-tune the final spirit’s strength.


In our next installment, we’ll examine how each component of the still influences the distillation process.